Description
In this experiment we are mimicking the part of the Earth’s water cycle in which evaporated water (water vapour) cools and condenses, forming cloud as they connect with dust. In this demonstration the alcohol acts as the dust, providing something cool for water droplets to attach to.
When the bottle is pressurised by pumping air into it, the air molecules collide with each other and warm the bottle and its contents. Releasing the pressure causes the water vapour to condense quickly, forming a cloud.
What’s in the kit?
- Cork stopper with inflation needle
- 70% Rubbing Alcohol
What you need:
- 1-2 L, PET bottle
- Stirrup pump
- Safety Glasses
Secrets for Success
- Using the atomiser bottle pump 5 squirts of rubbing alcohol into your PET bottle (label removed).
- Push in the cork stopper – the further it is pushed in the more air will be needed to be pumped in to build up a high enough pressure to push it out.
- Attach the pump to the inflation needle.
- Hold the bottle with the cork facing away from you. Make sure you have a clear space of at least 1 m in front of you – the cork will leave the bottle at a very high speed.
- Get your volunteer to stand beside you with one foot on the stirrup pump base. They then have to pull the handle to its maximum height and then push down as fast as they can until the bottle and cork separate.
- You will end up with a fantastic cloud in the bottle.
Science in a Nutshell
In this experiment we are mimicking the part of the Earth’s water cycle in which evaporated water (water vapour) cools and condenses, forming cloud as they connect with dust. In this demonstration the alcohol acts as the dust, providing something cool for water droplets to attach to.
When the bottle is pressurised by pumping air into it, the air molecules collide with each other and warm the bottle and its contents. Releasing the pressure causes the water vapour to condense quickly, forming a cloud.
Bonus: Reverse the experiment!
Before the cloud disappears, put the cork back in and pump a couple more times. The cloud will disappear as quickly as it came. Release the pressure, and the cloud will form again.
How do real clouds form?
Clouds require three things to form:
- Water molecules
- Cloud condensation nuclei – dust or air pollution
- Temperature or pressure changes
Water molecules are in the air all around us, even though we can’t normally see them. Normally, these water molecules bounce around.
Real clouds form when warm air rises in the atmosphere and cools down. Cloud condensation nuclei, such as small particles of dust and pollution, enable water molecules to stick together and stop bouncing around. The water molecules condense around the nuclei to form clouds. Clouds are just groups of tiny water droplets that stick together around cloud condensation nuclei when temperatures are low.
High- and low-pressure systems cause day-to-day changes in our weather.
Ascending and descending air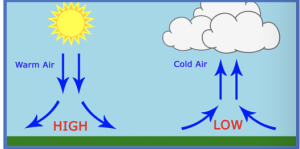
Areas of high and low pressure are caused by ascending and descending air. As air warms it ascends, leading to low pressure at the surface. As air cools it descends, leading to high pressure at the surface.
In general, low pressure leads to unsettled weather conditions and high pressure leads to settled weather conditions.
Anticyclone (high pressure)
In an anticyclone (high pressure) the winds tend to be light and blow in a clockwise direction (in the northern hemisphere). Also, the air is descending, which reduces the formation of cloud and leads to light winds and settled weather conditions.
Depression (low pressure)
In a depression (low pressure), air is rising and blows in an anticlockwise direction around the low (in the northern hemisphere). As it rises and cools, water vapour condenses to form clouds and perhaps precipitation. This is why the weather in a depression is often unsettled, there are usually weather fronts associated with depressions.








